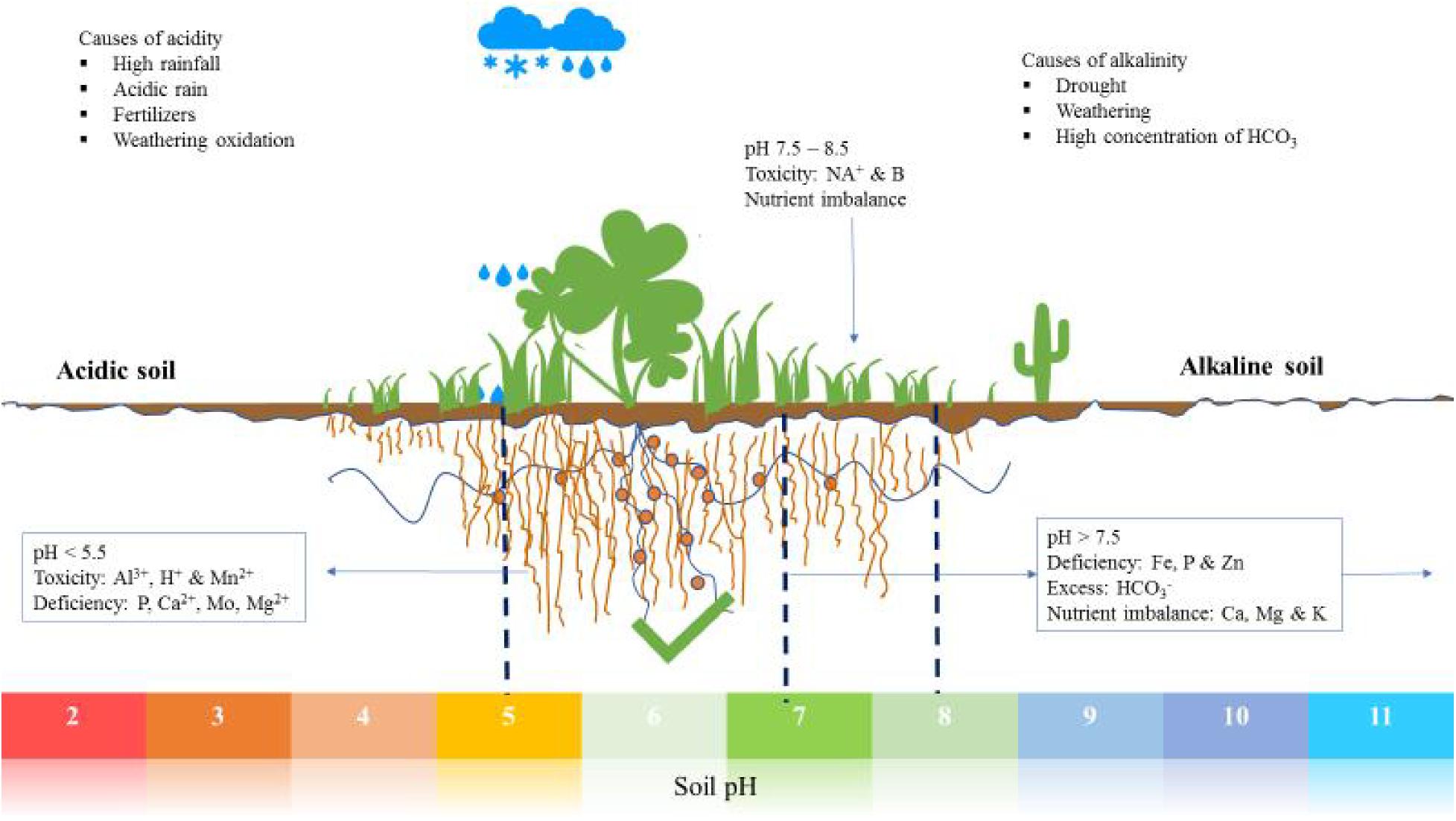
Soil pH levels critically influence plant nutrient availability and root growth. Optimal pH ranges are vital for healthy plant development.
soil pH is essential for gardeners and farmers, as it affects how plants absorb nutrients. Soil pH measures its acidity or alkalinity, which can significantly impact plant health. Most plants thrive in slightly acidic to neutral soils (pH 6-7.
5), where essential nutrients like nitrogen, phosphorus, and potassium are most accessible. Outside this range, plants may struggle to thrive as toxic elements can become more soluble, and nutrients may become locked up, unavailable for plant uptake. Regular soil testing allows gardeners to amend the soil accordingly, ensuring it meets the specific pH requirements of the plants they wish to grow. Balancing the soil pH is a fundamental step toward achieving a vibrant and productive garden or crop yield.
Credit: www.frontiersin.org
The Crucial Role Of Soil Ph In Plant Health
Garden success starts beneath our feet. A healthy plant’s journey begins with the soil it calls home. One of the most important aspects of soil health is its pH level. This simple measure can mean the difference between a flourishing garden and a failing one.
Defining Soil Ph
Soil pH is a measure of acidity or alkalinity in the ground. It ranges from 0 to 14, with 7 as neutral. Numbers below 7 indicate acidic soil, while above 7 means the soil is alkaline. Each plant has a preferred pH range to maximize growth and health.
How Soil Ph Affects Plant Nutrient Availability
Soil pH plays a pivotal role in nutrient uptake by plants. It influences the chemical form of nutrients in the soil, thus affecting their availability. Some nutrients are more soluble, hence available to plants, at certain pH levels. Incorrect pH can lead to deficiencies or toxicities, harming plant growth.
| pH Level | Nutrient Availability |
|---|---|
| Acidic Soil (pH < 7) | Iron, Manganese, and other micro-nutrients become more available, which can be good for certain plants but harmful if levels are too high. |
| Neutral Soil (pH ≈ 7) | Most nutrients are optimally available. This range is ideal for a wide variety of plants, contributing to robust growth. |
| Alkaline Soil (pH > 7) | Nutrients like Iron, Zinc, and Manganese may become less available, which can inhibit plant growth and lead to yellowing leaves. |
- Acid-loving plants such as azaleas thrive in lower pH.
- Vegetables like broccoli and cabbage favor a slightly acidic to neutral pH.
- Very alkaline soils might require amendments to lower the pH for certain crops.
Testing soil pH is easy and correcting it can be as simple as adding lime to raise or sulfur to lower the pH. Understanding and adjusting soil pH appropriately ensures that plants have access to the nutrients they need for optimal health and productivity.
Acidic Versus Alkaline Soils
Welcome to the vibrant world of soil pH where plant health begins. Soil pH measures its acidity or alkalinity. It is a key factor in the well-being of plants. The pH scale ranges from 0 to 14. A soil with a pH less than 7 is acidic. When the pH is above 7, the soil is alkaline. Most plants thrive in a pH range of 6 to 7.5. Yet, some plants favor acidic or alkaline conditions. Let’s dig deeper into these two types of soils.
Characteristics Of Acidic Soils
Acidic soils, also known as ‘sour’ soils, have a pH of less than 7. They naturally occur in areas with high rainfall. Acidic soils can limit plant growth. This happens due to the lower availability of key nutrients. Over-acidity may cause toxicity of elements like aluminum. Here are some characteristics:
- Lower pH levels, often below 6.5
- Frequent in areas with heavy rainfall and forested regions
- May contain higher levels of soluble aluminum and manganese
- Limited availability of calcium, magnesium, and phosphorus
Rhododendrons and blueberries thrive in acidic soils. A soil test can reveal the pH and suggest amendments to balance it.
Characteristics Of Alkaline Soils
Alkaline soils, also known as ‘sweet’ soils, have a pH above 7. They often result from high limestone content. Alkaline conditions can lead to nutrient deficiencies. Here’s what sets alkaline soils apart:
| Characteristic | Description |
|---|---|
| Higher pH levels | Typically above 7.5 |
| Common in arid and semi-arid regions | Low rainfall prevents leaching away of minerals |
| Deficiencies | Iron, zinc, and manganese may become less available |
| Mineral Content | Often rich in calcium and magnesium |
Plants like lavender and clematis prefer alkaline soils. Testing soil pH could pave the way for healthier plants. Balancing soil pH might require adding sulfur or organic matter.
Optimal Ph Ranges For Common Plants
Every plant thrives best at a certain soil pH level. The health of our favorite greens depends on getting this balance right. pH levels range from 0 to 14. Soil with a pH less than 7 is acidic. Soil with a pH greater than 7 is alkaline. Just the right pH level means the best crop yields, gorgeous flowers, and the happiest gardens.
Favorite Ph Levels For Vegetable Crops
Vegetables each have their own pH sweet spot. This determines their growth, nutrient uptake, and resilience. Here’s a peek at the pH preferences of some common veggies:
| Vegetable | Preferred pH Level |
|---|---|
| Tomatoes | 6.0 – 6.8 |
| Carrots | 5.8 – 7.0 |
| Peppers | 5.5 – 7.0 |
| Spinach | 6.0 – 7.5 |
| Onions | 6.0 – 6.8 |
Flowering Plants And Their Preferred Ph
Flowering plants bring color and joy to any space. They need the right pH to bloom in all their glory. Below is a simple guide for common flowering plants:
- Roses: prefer a pH of 6.0 – 6.5
- Azaleas: love a more acidic soil, around 4.5 – 6.0
- Marigolds: flexible but favor a pH of 6.0 – 7.5
- Lavender: thrives in a pH of 6.5 – 7.5
- Begonias: best in a slightly acidic pH of 5.5 – 6.2

Symptoms Of Ph Imbalance In Plants
Soil pH is crucial for plant health. It affects nutrient availability and root growth. Symptoms of pH imbalance in plants can lead to poor growth and yield. Let’s identify the signs of distress linked to pH levels.
Signs Of Acidic Soil Stress
Plants struggling in acidic soil may show these symptoms:
- Leaf yellowing (chlorosis), especially in new leaves.
- Poor root development, limiting water and nutrient uptake.
- Leaf tip burn or brown spots, showing nutrient deficiencies.
- Stunted growth of both shoots and roots.
- Moss or weed overgrowth, which thrives in acidic conditions.
These signs alert gardeners to test the soil pH and adjust as needed.
Signs Of Alkaline Soil Stress
In contrast, plants in alkaline soil frequently exhibit:
- Slow growth due to limited nutrient availability.
- Browning of leaf edges, often a sign of potassium deficiency.
- Weakened stems and underdeveloped foliage.
- Iron deficiency, shown by a yellowing between green leaf veins.
- Pale flower colors and fewer blooms.
Stunted seedling growth or failure to thrive can also indicate alkaline soil issues.
Adjusting Soil Ph For Plant Health
Soil pH is key to healthy plants. It affects nutrient availability. Plants need the right pH to thrive. Incorrect pH can cause problems. Healthy soil means healthy plants.
Methods To Raise Soil Ph
A low pH means soil is acidic. Some plants may struggle. We can fix this. Here are methods to raise soil pH:
- Lime: Adds calcium and magnesium. It reduces soil acidity.
- Dolomite lime: Like lime but with more magnesium.
- Wood ash: Rich in potassium. It can increase pH.
- Baking soda: A quick, mild way to raise pH.
Methods To Lower Soil Ph
Some soils are too alkaline. High pH affects nutrient uptake. Here’s how to lower soil pH:
- Sulfur: Adds acidity over time. It’s effective.
- Aluminum sulfate: Acts faster than sulfur.
- Iron sulfate: Adds iron. It also reduces pH quickly.
- Organic mulches: Pine needles or peat moss can help.
Soil Ph Testing And Monitoring
Understanding the acidity or alkalinity of soil, known as soil pH, is vital to plant health. Soil pH directly affects nutrient availability and the life of beneficial microorganisms. Testing and monitoring soil pH ensures plants thrive in their growing environment.
How To Test Your Soil’s Ph
Testing your soil’s pH is a simple process that you can do at home. Begin by collecting a soil sample from several areas in your garden to get an average pH reading. Use these steps:
- Remove the top layer of soil.
- Collect a soil sample from 4-6 inches deep.
- Mix soil from different areas to form a composite sample.
- Let the soil dry at room temperature.
- Use a pH test kit or pH meter for the reading.
DIY test kits are available at garden stores, but for more precise results, consider a professional lab test.
Frequency And Timing Of Soil Ph Testing
Maintain optimal soil conditions by regular pH testing. The frequency depends on several factors:
| Type of Plants | Testing Frequency |
|---|---|
| High-maintenance crops | Annually |
| Stable perennial gardens | Every 2-3 years |
Time your testing when soil is relatively stable—typically in the spring or fall. Before planting new crops, adjust the soil pH if necessary to provide an ideal growing environment.
The Connection Between Soil Ph And Microorganisms
The health of a garden lies beneath the surface, in the world of tiny creatures within the soil. Soil pH greatly influences this underground world, particularly the microorganisms that are vital to plant growth. Understanding this connection can unlock the secret to a thriving garden.
Microbial Activity In Various Ph Levels
Soil pH measures its acidity or alkalinity on a scale from 0 to 14. Microorganisms thrive in different pH ranges. For instance:
- Bacteria prefer neutral to slightly alkaline soils, typically between pH 6.5 and 7.5.
- Fungi are more tolerant of acidity, with optimal activity around pH 5.5 to 6.5.
- Actinomycetes, like bacteria, enjoy neutral soils.
Soil with the wrong pH can reduce microbial activity. This slows down the processes these tiny helpers perform.
Impact Of Soil Ph On Decomposition And Nutrient Cycling
| pH Level | Decomposition | Nutrient Availability |
|---|---|---|
| Low pH (< 6.0) | Slows down, affecting organic matter breakdown | Nutrients like phosphorus become less available |
| Neutral pH (~ 7.0) | Optimized, promoting efficient recycling of nutrients | Most nutrients are readily available for plants |
| High pH (> 7.5) | Can hinder microbe activity, slowing decomposition | Iron, manganese, and other micronutrients become less accessible |
A balanced pH ensures healthy decomposition and nutrient cycling. This process feeds plants and keeps them growing strong. By maintaining the right pH balance, gardeners aid microorganisms in breaking down organic matter, releasing vital nutrients back into the soil.
Best Practices For Maintaining Ideal Soil Ph

Best practices for maintaining ideal soil pH are crucial for plant growth and health. Soil pH affects nutrient availability, soil bacteria, and the overall structure of the soil. It’s key to match your soil’s pH to the preferences of your plants. Let’s explore how you can do this effectively.
Organic Amendments And Ph Management
Using organic matter helps manage soil pH levels. Adding materials like compost, manure, or leaf mold can make a big difference. These amendments increase the soil’s organic content and slowly adjust the pH.
- Compost: Improves drainage and neutrality
- Manure: Raises pH in acidic soils
- Leaf mold: Adds acidity for certain crops
Regular Amendments Versus One-time Corrections
Understanding the difference between regular amendments and one-time applications is key. Regular amendments, like compost, are applied yearly. One-time corrections, like lime or sulfur, adjust pH quickly.
| Amendment Type | Function | Application Rate |
|---|---|---|
| Organic | Slowly alters pH | Regularly as needed |
| Lime/Sulfur | Quick pH change | Specific to soil test |
Test your soil regularly to know if you need a one-time correction or regular maintenance. Keep a record of the pH to track the soil’s health over time.
Credit: www.rainpointonline.com
Frequently Asked Questions Of Importance Of Soil Ph To Plants
How Is The Soil Ph Important To Plants?
Soil pH affects nutrient availability, with extremes inhibiting plant growth. Ideal pH ensures nutrients are accessible, allowing healthy plant development.
What Are The 5 Importance Of Ph In Soil?
Soil pH affects nutrient availability, with extreme levels limiting plant uptake. It influences soil microbial activity, impacting nutrient cycling and plant health. PH impacts soil structure, affecting aeration and water movement. It dictates herbicide efficacy, with some requiring specific pH conditions.
Lastly, pH plays a role in metal toxicity, where certain pH levels can increase or decrease the risk.
What Happens To Plants If The Soil Ph Is Too High?
High soil pH can lead to nutrient deficiencies, harming plant growth and causing yellowing of leaves or stunted development.
What Is The Most Important Effect Of Soil Ph On Plant Growth Its Influence On?
Soil pH crucially affects nutrient availability and uptake by plants, influencing their health and growth.
Conclusion
Understanding soil pH is crucial for any gardener or farmer’s success. It directly influences plant growth, nutrient availability, and overall plant health. By monitoring and adjusting soil pH, we can ensure that our plants thrive. Remember, the right pH balance can lead to a bountiful, vibrant garden.
Keep your soil in check for happy, healthy plants!






2 Responses